Porous poly(2-hydroxyethyl methacrylate) hydrogels synthesized within high internal phase emulsions
Abstract
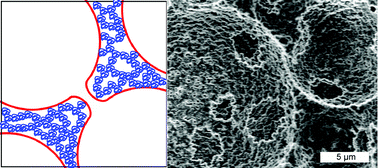
Hydrogels, such as those based on poly(2-hydroxyethyl methacrylate) (PHEMA), are hydrophilic three dimensional network structures that undergo extensive swelling in water. PolyHIPEs are highly porous, crosslinked polymers typically synthesized within high internal phase emulsions (HIPEs). This research describes materials with enhanced water absorption that combine hydrogel water absorption with capillary action by synthesizing PHEMA-based polyHIPEs within oil-in-water HIPEs. The variation in the N,N-methylenebisacrylamide (MBAM) crosslinking comonomer content yields a narrow synthesis window in which water-swollen micro-gel particles phase separate, agglomerate, and form a heterogeneous polyHIPE wall structure with nanoscale porosity. Surprisingly, a hydrogel polyHIPE with a relatively high MBAM content also had the highest surface area and the highest water absorption. Ultimately, it is the influence of the MBAM content on the polymer hydrophilicity and on the porous structure that determines its effects on the properties.

 Please wait while we load your content...
Please wait while we load your content...