Increasing dimethyl ether production from biomass-derived syngas via sorption enhanced dimethyl ether synthesis
Abstract
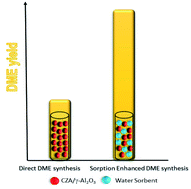
The direct synthesis of dimethyl ether (DME) from biomass-derived syngas is a topic of great interest in the field of biofuels. The process takes place in one reactor combining two catalytic functions, Cu/ZnO/Al2O3 (CZA) for the synthesis of methanol and an acid catalyst (typically γ-Al2O3) for methanol dehydration to DME. However, the catalytic performance of those catalysts is negatively affected by the high CO2/CO ratio in the bio-syngas, resulting in low methanol and DME production rates. In this work, we show that promoters such as zirconium and gallium oxides increase the CO fraction in the syngas. However, the production of H2O is also increased, leading to the deactivation of both CZA and γ-Al2O3. The addition of a water sorbent (zeolite 3A) in the reaction medium alleviates the detrimental effect of H2O in the direct synthesis of DME from CO2-rich syngas. Thus, DME production over the CZA/γ-Al2O3 catalytic bed increases from ca. 8.7% to 70% when a zeolite 3A is placed in the reaction medium. In fact, carbon conversions higher than conventional equilibrium conversions are achieved. This work demonstrates that the sorption enhanced synthesis of DME is a suitable strategy to increase DME production from biomass-derived syngas.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...