Titanium nitride-supported Cu–Ni bifunctional electrocatalysts for CO2 reduction and the oxygen evolution reaction†
Abstract
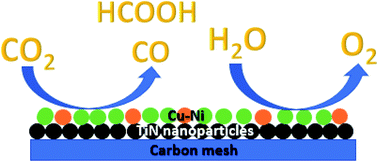
This work reports the fabrication of a bifunctional electrocatalyst capable of the CO2 reduction reaction (CO2RR) and the O2 evolution reaction (OER). The catalyst was fabricated by the electrodeposition of a Cu–Ni alloy onto titanium nitride (TiN) nanoparticles on carbon mesh. The use of TiN on carbon as a solid support greatly enhances both the CO2 reduction and O2 evolution catalytic activity of the Cu–Ni particles. By combining both the CO2RR and OER in a two-electrode device, this bifunctional system exhibited good durability for 24 hours at an overpotential of 670 mV at 2 mA cm−2. In this configuration, this device produced 19.1% CO and 6.7% formate as products of the CO2RR. Through cyclic voltammetry studies, it was found that Cu–Ni preferentially deposits on TiN as compared to bare carbon, thus allowing for more Cu–Ni to be uniformly deposited, which significantly enhances catalysis. Current densities normalized to the amount of the Cu–Ni catalyst demonstrate that Cu–Ni on TiN/C is significantly more active per gram of catalyst. X-ray diffraction studies suggest that the Cu (111) and Ni (111) facets contribute to the enhanced catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...