The use of supercritical water for the catalyst-free oxidation of coarse aluminum for hydrogen production
Abstract
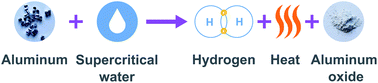
Maximizing the use of renewable resources requires clean, sustainable and recyclable energy carriers for energy trade and long-term storage. Aluminum is energy dense, plentiful, and recyclable and, when reacted with water, the stored energy is released as hydrogen and heat. In this study, we investigated the use of high-temperature liquid water and supercritical water as oxidizers for coarse aluminum. We performed experiments using a variety of aluminum morphologies, including coarse aluminum pieces measuring up to 3 mm in diameter, and water ranging in temperature from 475 K to 650 K (and the corresponding saturated vapour pressures). Previous studies of aluminum–water reactions have focused on low temperature experiments using catalysts, specialized alloys, or nano-powders to increase reaction efficiency. These low-temperature approaches have been shown to be effective but add complexity and expense and waste the thermal energy of the reaction. Our results show that, without special measures, 100% hydrogen yield is possible from coarse aluminum particles and scrap aluminum, when the reaction temperature and pressure are increased. A change in reaction efficiency was observed at 550 K. Up to this temperature, the 55 μm and 120 μm powders had yields below 30%, the aluminum slugs and 2 mm plate had yields close to zero. At temperatures between 550 K and the supercritical temperature, there was a marked increase in hydrogen yield. At temperatures above 647 K and pressures above 220 bar, the critical point of water, 100% of the theoretical hydrogen yield was achieved across all samples tested. These findings open the door to using aluminum as a recyclable energy carrier for renewable energy.


 Please wait while we load your content...
Please wait while we load your content...