A nickel and cobalt bimetal organic framework with high capacity as an anode material for lithium-ion batteries†
Abstract
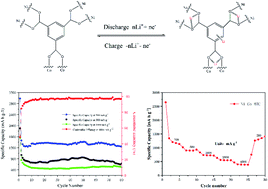
Owing to the high controllability and regular pore structure, metal–organic frameworks (MOFs) have been considered as promising electrode materials. In this work, we report the synthesis of a nickel and cobalt 1,3,5-benzenetricarboxylate bimetal organic framework (Ni–Co-BTC) using a common solvothermal method, and investigate its electrochemical performance as an anode material for lithium-ion batteries. The Ni–Co-BTC anode shows a high specific capacity of 1242 mA h g−1 at a current density of 200 mA g−1 in the potential window of 0.01–3.0 V versus Li/Li+ along with excellent rate performance (384 mA h g−1 at a current density of 4 A g−1), which could be ascribed to the conjugated carboxylates with a stronger π–π interaction and the synergistic effect of two cations. In addition, the mechanism of Li storage demonstrated that Li+ inserts into (or extracts from) the carboxylate groups and the benzene ring without the direct engagement of metal ions during galvanostatic charge–discharge measurements. More importantly, the Ni–Co-BTC MOF will be regarded as one of the promising anode materials for advanced Li storage.


 Please wait while we load your content...
Please wait while we load your content...