Boric acid: the first effective inorganic promoter for methane hydrate formation under static conditions†
Abstract
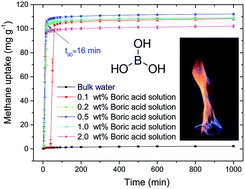
We first demonstrated that boric acid, an inexpensive and easily handled inorganic compound, can be used to dramatically enhance methane uptake kinetics in the formation of methane hydrate under static conditions. Furthermore, we revealed the synergistic effects between inorganic and organic promoters on the methane hydrate formation with a gravimetric capacity up to 146 mg g−1 (approximately 97 wt% of water was converted into hydrate). We also showed that sodium disilicate, a more cost-effective inorganic salt, can be employed as an effective promoter for the formation of methane hydrate.


 Please wait while we load your content...
Please wait while we load your content...