An aqueous rechargeable dual-ion hybrid battery based on Zn//LiTi2(PO4)3 electrodes†
Abstract
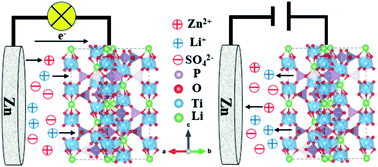
Aqueous rechargeable lithium ion batteries (ARLIBs) have attracted wide attention in the energy storage field due to their nontoxicity and high safety. Nevertheless, they face many challenges relating to capacity and stability due to the restricted selection of anode materials that can act within the narrow stable potential window of water. Herein, we developed a highly reversible Zn//LiTi2(PO4)3@C dual-ion hybrid battery, where the LiTi2(PO4)3@C material was synthesized via a facile sol–gel method and used as the cathode. A Zn sheet was chosen as the anode, and a solution consisting of 0.5 M ZnSO4 and 0.25 M Li2SO4 was the electrolyte. This aqueous rechargeable dual-ion hybrid battery exhibited stable cycling performance and excellent rate performance. After 500 cycles at a high current density of 12C (1C = 138 mA g−1), the reversible discharge capacity was 49 mA h g−1, not much less than the initial capacity. In a rate test, the discharge capacity rebounded to a value of 60 mA h g−1 when the current density was returned from 18C back to 12C after 10 cycles. This battery system may provide great insight into designing dual-ion hybrid battery systems with high rate performances.


 Please wait while we load your content...
Please wait while we load your content...