An experimental high temperature thermal battery coupled to a low temperature metal hydride for solar thermal energy storage
Abstract
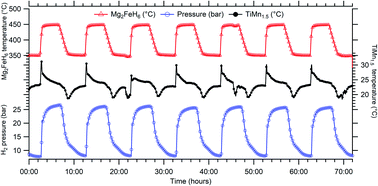
Metal hydrides have demonstrated ideal physical properties to be the next generation of thermal batteries for solar thermal power plants. Previous studies have demonstrated that they already operate at the required operational temperature and offer greater energy densities than existing technology. Thermal batteries using metal hydrides need to store hydrogen gas released during charging, and so far, practical demonstrations have employed volumetric storage of gas. This practical study utilises a low temperature metal hydride, titanium manganese hydride (TiMn1.5Hx), to store hydrogen gas, whilst magnesium iron hydride (Mg2FeH6) is used as a high temperature thermal battery. The coupled system is able to achieve consistent energy storage and release cycles. With titanium manganese hydride operating at ambient temperature (20 °C), Mg2FeH6 has to operate between ∼350 °C and ∼500 °C to counteract the pressure hysteresis displayed by TiMn1.5 between hydrogen uptake and release. The results attest the high susceptibility of both materials to thermal issues, such as a requirement for large temperature offsets, in order for the battery to achieve full cycling capacity. An energy density of 1488 kJ kg−1 was experimentally attained for 40 g of Mg2FeH6 with a maximum operating temperature around 520 °C.


 Please wait while we load your content...
Please wait while we load your content...