Cerium and nitrogen doped CoP nanorod arrays for hydrogen evolution in all pH conditions†
Abstract
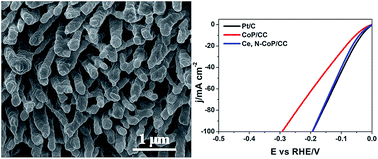
Exploring pH-universal electrocatalysts for hydrogen evolution has attracted focused research attention. Heteroatom doping is recognized as an effective solution for activating the catalytic activity of transition metal-based electrocatalysts. Herein, cerium with 4f electrons and nitrogen were firstly selected as guest atoms to be doped into the CoP nanorod array host catalyst with the aim to produce efficient nonprecious-metal based catalysts for hydrogen evolution in all pH conditions. The electrocatalytic measurements proved that Ce and N co-doping endows CoP with remarkably improved intrinsic catalytic activity and kinetics, affording a current of 10 mA cm−2 at the low overpotentials of 66 mV, 72 mV, and 41 mV in 0.5 M H2SO4, 1 M phosphate buffer, and 1 M KOH, respectively. Such catalytic performances prove that the Ce and N doped CoP nanorod array is a promising pH-tolerant catalyst.
- This article is part of the themed collection: 2019 Sustainable Energy and Fuels HOT Articles


 Please wait while we load your content...
Please wait while we load your content...