Why nitrogen favors oxygen reduction on graphitic materials†
Abstract
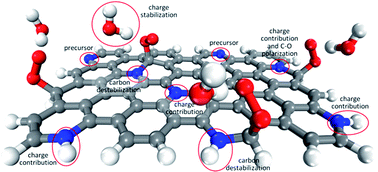
Nitrogen-doped graphitic materials as promising catalysts for the oxygen reduction reaction in fuel-cells have been mainly investigated under the graphitic versus pyridinic nitrogen-dopant dichotomy approach. However, we show here that the active sites, reaction mechanism, selectivity and even the origin of each behavior can be better understood when the stability of the possible active site and the eventual contribution of charge from the surface are considered separately. The roles in the reaction played by specific nitrogen-dopants, the hydrogenation of pyridinic nitrogen-dopants and the solvation effect are all clarified. The investigated activity is much more closely linked to the edges, where certain carbon atoms are sufficiently unstable or can be destabilized by means of adjacent nitrogen-dopants, and where reaction intermediates can be better relaxed, than to the presence of specific nitrogen-dopants. Unfortunately, high overpotentials and the undesired production of hydrogen peroxide appear to be unavoidable in the oxygen reduction to water on these materials.


 Please wait while we load your content...
Please wait while we load your content...