Photocatalytic hydrogen evolution from neutral aqueous solution by a water-soluble cobalt(ii) porphyrin†
Abstract
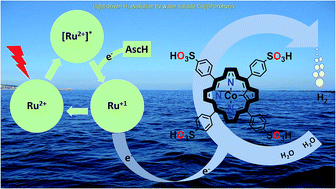
Efficient storage of solar energy via light-driven hydrogen evolution is an attractive and promising strategy to address challenges related to increasing global energy demand. Herein, we report a highly active homogeneous photocatalytic hydrogen evolution system comprising a water-soluble cobalt(II) porphyrin, CoTPPS, as a molecular catalyst, [Ru(bpy)3]2+ as a photosensitizer, and ascorbic acid as a sacrificial electron donor. Under optimized conditions, a hydrogen evolution rate of 1.3 μmol min−1, a TOF of 120.8 min−1, and a TON of 6410 are obtained in 1 M phosphate buffer at pH 6.8 by irradiation with an LED light source at 420 nm and 0.6 W cm−2. The studies on the effects of pH and catalyst concentration showed that optimal H2 evolution efficiency was achieved at a pH of 6.8 and a catalyst concentration of 1.5 μM. Moreover, the kinetics of the fluorescence emission quenching experiment indicates that the rate of reductive quenching of [Ru(bpy)3]2+* by ascorbate (7.5 × 106 s−1) is higher than that of oxidative quenching by CoTPPS (1.7 × 104 s−1). As also supported by redox potential energy level analysis, a mechanistic pathway was proposed in which reduction of photo-excited [Ru(bpy)3]2+* by ascorbic acid produces [Ru(bpy)3]1+, which is a strong reductant and will subsequently reduce CoTPPS to generate cobalt-hydride for hydrogen evolution after protonation.
- This article is part of the themed collection: Artificial Photosynthesis - From Sunlight to Fuels and Valuable Products for a Sustainable Future


 Please wait while we load your content...
Please wait while we load your content...