Photocatalytic hydrogen evolution driven by platinated CdS nanorods with a hexacyanidoruthenate redox mediator†
Abstract
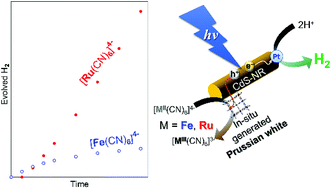
We have investigated the effect of hexacyanidometallate [M(CN)6]4− (M = Fe or Ru) redox mediators on the photocatalytic H2 evolution reaction driven by Pt-cocatalyst-loaded cadmium sulfide nanorods (Pt/CdS-NR). A larger amount of H2 evolved under blue LED light irradiation (λ = 470 ± 10 nm) in the presence of [Ru(CN)6]4− than in the presence of [Fe(CN)6]4−, despite the more positive M(III)/M(II) redox potential of [Ru(CN)6]4− (0.89 V vs. NHE) than [Fe(CN)6]4− (0.36 V vs. NHE). PXRD and IR spectral experiments during the photocatalytic H2 evolution reaction clearly indicate that the Prussian white analogue K2[CdRu(CN)6] (CdRu-PW) was increasingly formed, producing a white precipitate, whereas only a trace amount of K2[CdFe(CN)6] (CdFe-PW) was formed in the reaction with [Fe(CN)6]4−. The amount of CdRu-PW produced during the photocatalytic H2 evolution reaction revealed that the electron source of H2 evolution is gradually changed from the S2− anions generated following the photocorrosion of CdS to [Ru(CN)6]4− because of effective hole transfer through the Ru(III)/Ru(II) redox step in the CdRu-PW (1.42 V vs. NHE) deposited on the CdS-NR surface. The colourless feature and more positive Ru(III)/Ru(II) redox potential of CdRu-PW than that of water oxidation suggest that CdRu-PW is a promising hole mediator for solar water splitting.


 Please wait while we load your content...
Please wait while we load your content...