Closing the carbon cycle to maximise climate change mitigation: power-to-methanol vs. power-to-direct air capture
Abstract
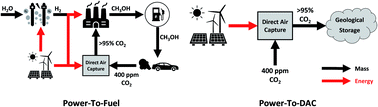
It is broadly recognised that CO2 capture and storage (CCS) and associated negative emissions technologies (NETs) are vital to meeting the Paris agreement target. The hitherto failure to deploy CCS on the required scale has led to the search for options to improve its economic return. CO2 capture and utilisation (CCU) has been proposed as an opportunity to generate value from waste CO2 emissions and improve the economic viability of CCS, with the suggestion of using curtailed renewable energy as a core component of this strategy. This study sets out to quantify (a) the amount of curtailed renewable energy that is likely to be available in the coming decades, (b) the amount of fossil CO2 emissions which can be avoided by using this curtailed energy to convert CO2 to methanol for use as a transport fuel – power-to-fuel, with the counterfactual of using that curtailed energy to directly remove CO2 from the atmosphere via direct air capture (DAC) and subsequent underground storage, power-to-DAC. In 2015, the UK curtailed 1277 GWh of renewable power, or 1.5% of total renewable power generated. Our analysis shows that the level of curtailed energy is unlikely to increase beyond 2.5% until renewable power accounts for more than 50% of total installed capacity. This is unlikely to be the case in the UK before 2035. It was found that: (1) power-to-DAC could achieve 0.23–0.67 tCO2 avoided MWh−1 of curtailed power, and (2) power-to-Fuel could achieve 0.13 tCO2 avoided MWh−1. The power-to-fuel concept was estimated to cost $209 tCO2 avoided−1 in addition to requiring an additional $430–660 tCO2 avoided−1 to finally close the carbon cycle by air capture. The power-to-DAC concept was found to cost only the $430–660 tCO2 avoided−1 for air capture. For power-to-fuel to become profitable, hydrogen prices would need to be less than or equal to $1635 tH2−1 or methanol prices must increase to $960 tMeOH−1. Absent this change in H2 price or methanol value, a subsidy of approximately $283 tCO2−1 would be required. A core conclusion of this study is that using (surplus) renewable energy for direct air capture and CO2 storage is a less costly and more effective option to mitigate climate change than using this energy to produce methanol to substitute gasoline.


 Please wait while we load your content...
Please wait while we load your content...