Understanding homogeneous hydrogen evolution reactivity and deactivation pathways of molecular molybdenum sulfide catalysts†
Abstract
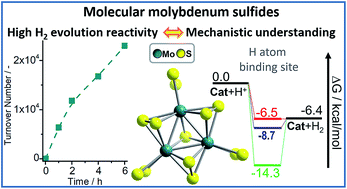
Molybdenum sulfides are highly active hydrogen evolution reaction (HER) catalysts based on earth abundant elements. Here, the molybdenum sulfide anion [Mo3S13]2− is used as a molecular model to rationalize HER reactivity of Mo–S-catalysts. For the first time, homogeneous, visible light-driven HER activity of [Mo3S13]2− is reported and high reactivity is observed (turnover number TON ∼23 000, maximum turnover frequency TOFmax ∼156 min−1). Experimental and theoretical studies shed light on the catalytic role of terminal disulfide ligands (S22−) and show that these ligands modulate catalyst redox-activity and electron transfer in solution. Partial substitution of the terminal disulfides with water ligands leads to the most active catalytic species, e.g. [Mo3S11(H2O)2]. In contrast, complete substitution of the terminal disulfides results in a significant loss of reactivity. These results could lay the foundations for the knowledge-based development of homogeneous and heterogeneous molybdenum sulfide catalysts.


 Please wait while we load your content...
Please wait while we load your content...