Single-crystal-to-single-crystal synthesis of a pseudostarch via topochemical azide–alkyne cycloaddition polymerization†
Abstract
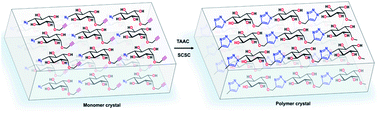
There is high demand for polysaccharide-mimics as enzyme-stable substitutes for polysaccharides for various applications. Circumventing the problems associated with the solution-phase synthesis of such polymers, we report here the synthesis of a crystalline polysaccharide-mimic by topochemical polymerization. By crystal engineering, we designed a topochemically reactive crystal of a glucose-mimicking monomer decorated with azide and alkyne units. In the crystal, the monomers arrange in head-to-tail fashion with their azide and alkyne groups in a ready-to-react antiparallel geometry, suitable for their topochemical azide–alkyne cycloaddition (TAAC) reaction. On heating the crystals, these pre-organized monomer molecules undergo regiospecific TAAC polymerization, yielding 1,4-triazolyl-linked pseudopolysaccharide (pseudostarch) in a single-crystal-to-single-crystal manner. This crystalline pseudostarch shows better thermal stability than its amorphous form and many natural polysaccharides.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...