Stereodivergent synthesis of β-iodoenol carbamates with CO2via photocatalysis†
Abstract
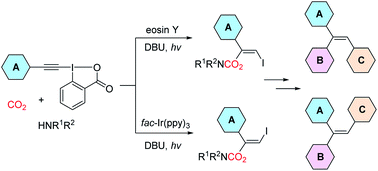
Photocatalytic conversion of carbon dioxide (CO2) into value-added chemicals is of great significance from the viewpoint of green chemistry and sustainable development. Here, we report a stereodivergent synthesis of β-iodoenol carbamates through a photocatalytic three-component coupling of ethynylbenziodoxolones, CO2 and amines. By choosing appropriate photocatalysts, both Z- and E-isomers of β-iodoenol carbamates, which are difficult to prepare using existing methods, can be obtained stereoselectively. This transformation featured mild conditions, excellent functional group compatibility and broad substrate scope. The potential synthetic utility of this protocol was demonstrated by late-stage modification of bioactive molecules and pharmaceuticals as well as by elaborating the products to access a wide range of valuable compounds. More importantly, this strategy could provide a general and practical method for stereodivergent construction of trisubstituted alkenes such as triarylalkenes, which represents a fascinating challenge in the field of organic chemistry research. A series of mechanism investigations revealed that the transformation might proceed through a charge-transfer complex which might be formed through a halogen bond.


 Please wait while we load your content...
Please wait while we load your content...