Transfer of polyantimony units†
Abstract
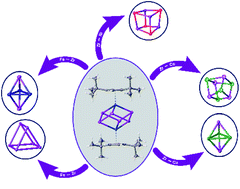
Transfer reagents are useful tools in chemistry to access metastable compounds. The reaction of [Cp′′2ZrCl2] with KSb(SiMe3)2 leads to the formation of the novel polyantimony triple decker complex [(Cp′′Zr)2(μ,η1:1:1:1:1:1-Sb6)] (1, Cp′′ = 1,3-di-tertbutyl-cyclopentadienyl), containing a chair-like Sb66− ligand. Compound 1 represents a valuable transfer reagent to form novel antimony ligand complexes. Thus, the reaction of 1 with CpR-substituted transition metal halides of nickel, cobalt and iron leads to the formation of a variety of novel Sbn ligand complexes, such as the cubane-like compounds [(Cp′′′Ni)4(μ3-Sb)4] (2) and [(Cp′′′Co)4(μ3-Sb4)] (3a) or the complexes [(CpBnCo)3(μ3-Sb)2] (4) and [(Cp′′′Fe)3(μ3-Sb)2] (5), representing a trigonal bipyramidal structure. Moreover, beside the transfer of Sb1 units, also the complete entity can be transferred as seen in the iron complex [(Cp′′′Fe)3(μ3,η4:4:4-Sb6)] (6). DFT calculations shed light on the bonding situation of the products.


 Please wait while we load your content...
Please wait while we load your content...