A mesoporous ionic solid with 272 Au I6Ag I3Cu II3 complex cations in a super huge crystal lattice†
Abstract
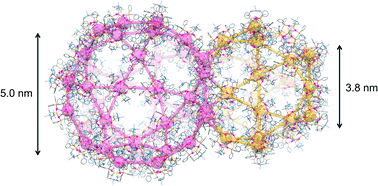
Here, we report a unique mesoporous ionic solid (I) generated from a cationic AuI6AgI3CuII3 dodecanuclear complex with D-penicillamine depending on the homochirality and crystallization conditions. I crystallizes in the cubic space group of F4132 with an extremely large cell volume of 2 171 340 Å3, containing 272 AuI6AgI3CuII3 complex cations in the unit cell. In I, the complex cations are connected to each other through CH⋯π interactions in a zeotype framework, the topology of which is the same as that of the metal–organic framework in MIL-101, with similar but much larger two types of polyhedral pores with internal diameters of 38.2 Å and 49.7 Å, which are occupied by counter-anions and water molecules. Due to the cationic nature of the framework, I undergoes quick, specific exchanges of counter-anions while retaining its single crystallinity. This study realized the creation of a non-covalent mesoporous framework from a single complex salt, providing a conceptual advance in solid chemistry and material science.


 Please wait while we load your content...
Please wait while we load your content...