Abstract
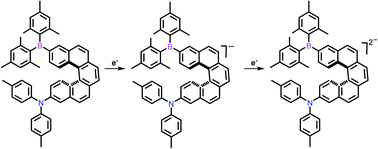
One-electron chemical reduction of 10-(dimesitylboryl)-N,N-di-p-tolylbenzo[c]phenanthrene-4-amine (3-B(Mes)2-[4]helix-9-N(p-Tol)2) 1 and 13-(dimesitylboryl)-N,N-di-p-tolyldibenzo[c,g]phenanthrene-8-amine (3-B(Mes)2-[5]helix-12-N(p-Tol)2) 2 gives rise to monoanions with extensive delocalization over the annulated helicene rings and the boron pz orbital. Two-electron chemical reduction of 1 and 2 produces open-shell biradicaloid dianions with temperature-dependent population of the triplet states due to small singlet-triplet gaps. These results have been confirmed by single-crystal X-ray diffraction, EPR and UV/vis-NIR spectroscopy, and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...