Catalytic enantioselective synthesis of benzocyclobutenols and cyclobutanols via a sequential reduction/C–H functionalization†
Abstract
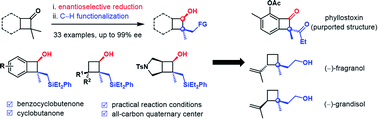
We report here a sequential enantioselective reduction/C–H functionalization to install contiguous stereogenic carbon centers of benzocyclobutenols and cyclobutanols. This strategy features a practical enantioselective reduction of a ketone and a diastereospecific iridium-catalyzed C–H silylation. Further transformations have been explored, including controllable regioselective ring-opening reactions. In addition, this strategy has been utilized for the synthesis of three natural products, phyllostoxin (proposed structure), grandisol and fragranol.


 Please wait while we load your content...
Please wait while we load your content...