Rigid, biconical hydrogen-bonded dimers that strongly encapsulate cationic guests in solution and the solid state†
Abstract
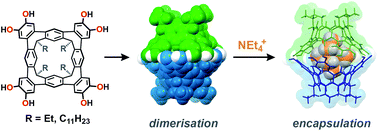
The octol of a new rigid, tetraarylene-bridged cavitand was investigated for self-assembly behaviour in solution. 1H and DOSY NMR spectroscopic experiments show that the cavitand readily dimerizes through an unusual seam of interdigitated hydrogen-bonds that is resistant to disruption by polar co-solvents. The well-defined cavity encapsulates small cationic guests, but not their neutral counterparts, restricting the conformation of sequestered tetraethylammonium in solution and the solid state.
- This article is part of the themed collection: Most popular 2021 supramolecular chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...