Probe metal binding mode of imine covalent organic frameworks: cycloiridation for (photo)catalytic hydrogen evolution from formate†
Abstract
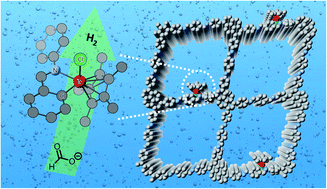
Metalation of covalent organic frameworks (COFs) is a critical strategy to functionalize COFs for advanced applications yet largely relies on the pre-installed specific metal docking sites in the network, such as porphyrin, salen, 2,2′-bipyridine, etc. We show in this study that the imine linkage of simple imine-based COFs, one of the most popular COFs, readily chelate transition metal (Ir in this work) via cyclometalation, which has not been explored before. The iridacycle decorated COF exhibited more than 10-fold efficiency enhancement in (photo)catalytic hydrogen evolution from aqueous formate solution than its molecular counterpart under mild conditions. This work will inspire more functional cyclometallated COFs to be explored beyond catalysis considering the large imine COF library and the rich metallacycle chemistry.


 Please wait while we load your content...
Please wait while we load your content...