Oxidative additions of alkynyl/vinyl iodides to gold and gold-catalyzed vinylation reactions triggered by the MeDalphos ligand†
Abstract
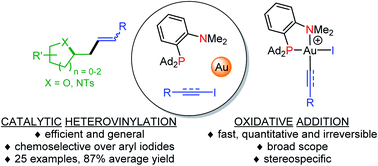
The hemilabile Ad2P(o-C6H4)NMe2 ligand promotes fast, quantitative and irreversible oxidative addition of alkynyl and vinyl iodides to gold. The reaction is general. It works with a broad range of substrates of various electronic bias and steric demand, and proceeds with complete retention of stereochemistry from Z and E vinyl iodides. Both alkynyl and vinyl iodides react faster than aryl iodides. The elementary step is amenable to catalysis. Oxidative addition of vinyl iodides to gold and π-activation of alkenols (and N-alkenyl amines) at gold have been combined to achieve hetero-vinylation reactions. A number of functionalized heterocycles, i.e. tetrahydrofuranes, tetrahydropyranes, oxepanes and pyrrolidines were obtained thereby (24 examples, 87% average yield). Taking advantage of the chemoselectivity for vinyl iodides over aryl iodides, sequential transformations involving first a hetero-vinylation step and then a C–N coupling, a C–C coupling or an heteroarylation were achieved from a vinyl/aryl bis-iodide substrate.
- This article is part of the themed collections: 2021 Chemical Science HOT Article Collection and Most popular 2021 main group, inorganic and organometallic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...