Difluorination of α-(bromomethyl)styrenes via I(I)/I(III) catalysis: facile access to electrophilic linchpins for drug discovery†
Abstract
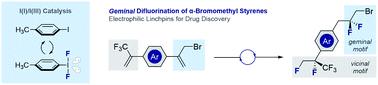
Simple α-(bromomethyl)styrenes can be processed to a variety of 1,1-difluorinated electrophilic building blocks via I(I)/I(III) catalysis. This inexpensive main group catalysis strategy employs p-TolI as an effective organocatalyst when combined with Selectfluor® and simple amine·HF complexes. Modulating Brønsted acidity enables simultaneous geminal and vicinal difluorination to occur, thereby providing a platform to generate multiply fluorinated scaffolds for further downstream derivatization. The method facilitates access to a tetrafluorinated API candidate for the treatment of amyotrophic lateral sclerosis. Preliminary validation of an enantioselective process is disclosed to access α-phenyl-β-difluoro-γ-bromo/chloro esters.


 Please wait while we load your content...
Please wait while we load your content...