Covalent and non-covalent albumin binding of Au(i) bis-NHCs via post-synthetic amide modification†
Abstract
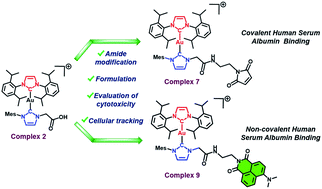
Recent decades have witnessed the emergence of Au(I) bis-N-heterocyclic carbenes (NHCs) as potential anticancer agents. However, these systems exhibit little interaction with serum proteins (e.g., human serum albumin), which presumably impacts their pharmacokinetic profile and tumor exposure. Anticancer drugs bound to human serum albumin (HSA) often benefit from significant advantages, including longer circulatory half-lives, tumor targeted delivery, and easier administration relative to the drug alone. In this work, we present Au(I) bis-NHCs complexes, 7 and 9, capable of binding to HSA. Complex 7 contains a reactive maleimide moiety for covalent protein conjugation, whereas its congener 9 contains a naphthalimide fluorophore for non-covalent binding. A similar drug motif was used in both cases. Complexes 7 and 9 were prepared from a carboxylic acid functionalized Au(I) bis-NHC (complex 2) using a newly developed post-synthetic amide functionalization protocol that allows coupling to both aliphatic and aromatic amines. Analytical, and in vitro techniques were used to confirm protein binding, as well as cellular uptake and antiproliferative activity in A549 human lung cancer cells. The present findings highlight a hitherto unexplored approach to modifying Au(I) bis-NHC drug candidates for protein ligation and serve to showcase the relative benefits of covalent and non-covalent HSA binding.


 Please wait while we load your content...
Please wait while we load your content...