Divergent reactivity of sulfinates with pyridinium salts based on one- versus two-electron pathways†
Abstract
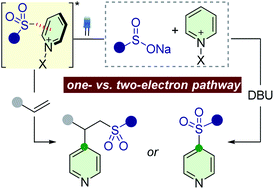
One of the main goals of modern synthesis is to develop distinct reaction pathways from identical starting materials for the efficient synthesis of diverse compounds. Herein, we disclose the unique divergent reactivity of the combination sets of pyridinium salts and sulfinates to achieve sulfonative pyridylation of alkenes and direct C4-sulfonylation of pyridines by controlling the one- versus two-electron reaction manifolds for the selective formation of each product. Base-catalyzed cross-coupling between sulfinates and N-amidopyridinium salts led to the direct introduction of a sulfonyl group into the C4 position of pyridines. Remarkably, the reactivity of this set of compounds is completely altered upon exposure to visible light: electron donor–acceptor complexes of N-amidopyridinium salts and sulfinates are formed to enable access to sulfonyl radicals. In this catalyst-free radical pathway, both sulfonyl and pyridyl groups could be incorporated into alkenes via a three-component reaction, which provides facile access to a variety of β-pyridyl alkyl sulfones. These two reactions are orthogonal and complementary, achieving a broad substrate scope in a late-stage fashion under mild reaction conditions.
- This article is part of the themed collection: Most popular 2021 organic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...