NHC-catalyzed covalent activation of heteroatoms for enantioselective reactions
Abstract
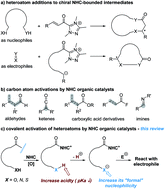
Covalent activation of heteroatoms enabled by N-heterocyclic carbene (NHC) organic catalysts for enantioselective reactions is evaluated and summarized in this review. To date, sulfur, oxygen, and nitrogen atoms can be activated in this manner to react with another substrate to construct chiral carbon–heteroatom bonds with high optical enantioselectivities. The activation starts with addition of an NHC catalyst to the carbonyl moiety (aldehyde or imine) of substrates that contain heteroatoms. The key in this approach is the formation of intermediates covalently bound to the NHC catalyst, in which the heteroatom of the substrate is activated as a nucleophilic reactive site.


 Please wait while we load your content...
Please wait while we load your content...