A controlled T7 transcription-driven symmetric amplification cascade machinery for single-molecule detection of multiple repair glycosylases†
Abstract
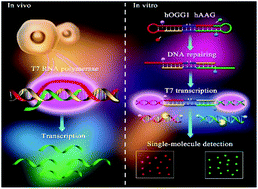
Genomic oxidation and alkylation are two of the most important forms of cytotoxic damage that may induce mutagenesis, carcinogenicity, and teratogenicity. Human 8-oxoguanine (hOGG1) and alkyladenine DNA glycosylases (hAAG) are responsible for two major forms of oxidative and alkylative damage repair, and their aberrant activities may cause repair deficiencies that are associated with a variety of human diseases, including cancers. Due to their complicated catalytic pathways and hydrolysis mechanisms, simultaneous and accurate detection of multiple repair glycosylases has remained a great challenge. Herein, by taking advantage of unique features of T7-based transcription and the intrinsic superiorities of single-molecule imaging techniques, we demonstrate for the first time the development of a controlled T7 transcription-driven symmetric amplification cascade machinery for single-molecule detection of hOGG1 and hAAG. The presence of hOGG1 and hAAG can remove damaged 8-oxoG and deoxyinosine, respectively, from the dumbbell substrate, resulting in breaking of the dumbbell substrate, unfolding of two loops, and exposure of two T7 promoters simultaneously. The T7 promoters can activate symmetric transcription amplifications with the unfolded loops as the templates, inducing efficient transcription to produce two different single-stranded RNA transcripts (i.e., reporter probes 1 and 2). Reporter probes 1 and 2 hybridize with signal probes 1 and 2, respectively, to initiate duplex-specific nuclease-directed cyclic digestion of the signal probes, liberating large amounts of Cy3 and Cy5 fluorescent molecules. The released Cy3 and Cy5 molecules can be simply measured by total internal reflection fluorescence-based single-molecule detection, with the Cy3 signal indicating the presence of hOGG1 and the Cy5 signal indicating the presence of hAAG. This method exhibits good specificity and high sensitivity with a detection limit of 3.52 × 10−8 U μL−1 for hOGG1 and 3.55 × 10−7 U μL−1 for hAAG, and it can even quantify repair glycosylases at the single-cell level. Moreover, it can be applied for the measurement of kinetic parameters, the screening of potential inhibitors, and the detection of repair glycosylases in human serum, providing a new paradigm for repair enzyme-related biomedical research, drug discovery, and clinical diagnosis.


 Please wait while we load your content...
Please wait while we load your content...