Anion-enhanced excited state charge separation in a spiro-locked N-heterocycle-fused push-pull zinc porphyrin†
Abstract
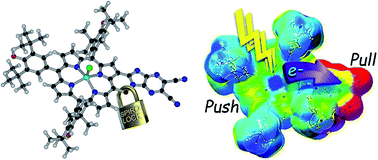
A new type of push–pull charge transfer complex, viz., a spiro-locked N-heterocycle-fused zinc porphyrin, ZnP-SQ, is shown to undergo excited state charge separation, which is enhanced by axial F− binding to the Zn center. In this push–pull design, the spiro-quinone group acts as a ‘lock’ promoting charge transfer interactions by constraining mutual coplanarity of the meso-phenol-substituted electron-rich Zn(II) porphyrin and an electron deficient N-heterocycle, as revealed by electrochemical and computational studies. Spectroelectrochemical studies have been used to identify the spectra of charge separated states, and charge separation upon photoexcitation of ZnP has been unequivocally established by using transient absorption spectroscopic techniques covering wide spatial and temporal regions. Further, global target analysis of the transient data using GloTarAn software is used to obtain the lifetimes of different photochemical events and reveal that fluoride anion complexation stabilizes the charge separated state to an appreciable extent.


 Please wait while we load your content...
Please wait while we load your content...