Studying the reactivity of alkyl substituted BODIPYs: first enantioselective addition of BODIPY to MBH carbonates†
Abstract
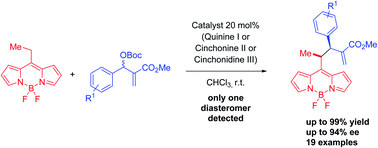
The first enantioselective addition of alkyl BODIPYs to Morita–Baylis–Hillman (MBH) carbonates is reported. This is the first reported enantioselective methodology using the methylene position of BODIPYs as a nucleophile. The reaction is efficiently catalyzed by cinchona alkaloids, achieving high enantioselectivities and total diastereoselectivity. The use of cinchona alkaloid pseudo enantiomers (chinine/cinchonine) allows us to obtain both pairs of enantiomers in similar yields and enantioselectivities, a common issue in this type of reaction. The photophysical study of these dyes (absorption and fluorescence) has been performed in order to determine their parameters and explore future possible application in bioimaging. In addition, electronic circular dichroism (ECD) studies supported by time-dependent density functional theory (TD-DFT) calculations were also performed.


 Please wait while we load your content...
Please wait while we load your content...