Boosting homogeneous chemoselective hydrogenation of olefins mediated by a bis(silylenyl)terphenyl-nickel(0) pre-catalyst†
Abstract
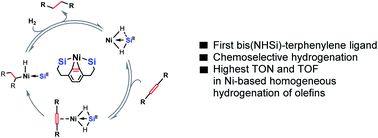
The isolable chelating bis(N-heterocyclic silylenyl)-substituted terphenyl ligand [SiII(Terp)SiII] as well as its bis(phosphine) analogue [PIII(Terp)PIII] have been synthesised and fully characterised. Their reaction with Ni(cod)2 (cod = cycloocta-1,5-diene) affords the corresponding 16 VE nickel(0) complexes with an intramolecular η2-arene coordination of Ni, [E(Terp)E]Ni(η2-arene) (E = PIII, SiII; arene = phenylene spacer). Due to a strong cooperativity of the Si and Ni sites in H2 activation and H atom transfer, [SiII(Terp)SiII]Ni(η2-arene) mediates very effectively and chemoselectively the homogeneously catalysed hydrogenation of olefins bearing functional groups at 1 bar H2 pressure and room temperature; in contrast, the bis(phosphine) analogous complex shows only poor activity. Catalytic and stoichiometric experiments revealed the important role of the η2-coordination of the Ni(0) site by the intramolecular phenylene with respect to the hydrogenation activity of [SiII(Terp)SiII]Ni(η2-arene). The mechanism has been established by kinetic measurements, including kinetic isotope effect (KIE) and Hammet-plot correlation. With this system, the currently highest performance of a homogeneous nickel-based hydrogenation catalyst of olefins (TON = 9800, TOF = 6800 h−1) could be realised.


 Please wait while we load your content...
Please wait while we load your content...