Catalytic asymmetric synthesis of N-substituted tetrahydroquinoxalines via regioselective Heyns rearrangement and stereoselective transfer hydrogenation in one pot†
Abstract
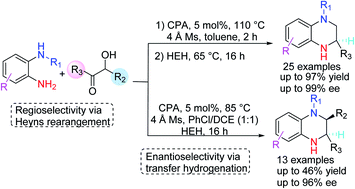
N-Substituted tetrahydroquinoxalines (37 examples) were step-economically obtained in good yield (<97%) and ee (<99%) with readily available substrates. The reaction proceeds through an interesting regioselective Heyns rearrangement/enantioselective transfer hydrogenation in one pot. The substrate scope and the reaction mechanism were systematically investigated.


 Please wait while we load your content...
Please wait while we load your content...