Iridium-catalyzed enantioselective olefinic C(sp2)–H allylic alkylation†
Abstract
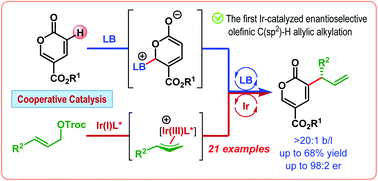
The first iridium-catalyzed enantioselective olefinic C(sp2)–H allylic alkylation is developed in cooperation with Lewis base catalysis. This reaction, catalyzed by cinchonidine and an in situ generated cyclometalated Ir(I)/phosphoramidite complex, makes use of the latent enolate character of an α,β-unsaturated carbonyl compound, namely coumalate ester, to introduce an allyl group at its α-position in a branched-selective manner in moderate to good yield with good to excellent enantioselectivities (up to 98 : 2 er).


 Please wait while we load your content...
Please wait while we load your content...