Mechanism of covalent binding of ibrutinib to Bruton's tyrosine kinase revealed by QM/MM calculations†
Abstract
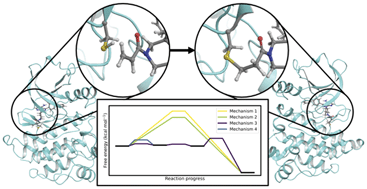
Ibrutinib is the first covalent inhibitor of Bruton's tyrosine kinase (BTK) to be used in the treatment of B-cell cancers. Understanding the mechanism of covalent inhibition will aid in the design of safer and more selective covalent inhibitors that target BTK. The mechanism of covalent inhibition in BTK has been uncertain because there is no appropriate residue nearby that can act as a base to deprotonate the cysteine thiol prior to covalent bond formation. We investigate several mechanisms of covalent modification of C481 in BTK by ibrutinib using combined quantum mechanics/molecular mechanics (QM/MM) molecular dynamics reaction simulations. The lowest energy pathway involves direct proton transfer from C481 to the acrylamide warhead in ibrutinib, followed by covalent bond formation to form an enol intermediate. There is a subsequent rate-limiting keto–enol tautomerisation step (ΔG‡ = 10.5 kcal mol−1) to reach the inactivated BTK/ibrutinib complex. Our results represent the first mechanistic study of BTK inactivation by ibrutinib to consider multiple mechanistic pathways. These findings should aid in the design of covalent drugs that target BTK and other similar targets.


 Please wait while we load your content...
Please wait while we load your content...