Site-selective functionalization of remote aliphatic C–H bonds via C–H metallation
Abstract
Directing group assistance provided a paradigm for controlling site-selectivity in transition metal-catalyzed C–H functionalization reactions. However, the kinetically and thermodynamically favored formation of 5-membered metallacycles has greatly hampered the selective activation of remote C(sp3)–H bonds via larger-membered metallacycles. Recent development to achieve remote C(sp3)–H functionalization via the C–H metallation process largely relies on employing specific substrates without accessible proximal C–H bonds. Encouragingly, recent advances in this field have enabled the selective functionalization of remote aliphatic C–H bonds in the presence of equally accessible proximal ones by taking advantage of the switch of the regiodetermining step, ring strain of metallacycles, multiple non-covalent interactions, and favourable reductive elimination from larger-membered metallacycles. In this review, we summarize these advancements according to the strategies used, hoping to facilitate further efforts to achieve site- and even enantioselective functionalization of remote C(sp3)–H bonds.
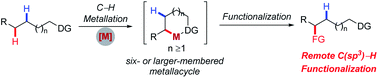


 Please wait while we load your content...
Please wait while we load your content...