Defining and navigating macrocycle chemical space†
Abstract
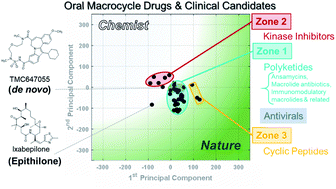
Macrocyclic compounds (MCs) are of growing interest for inhibition of challenging drug targets. We consider afresh what structural and physicochemical features could be relevant to the bioactivity of this compound class. Using these features, we performed Principal Component Analysis to map oral and non-oral macrocycle drugs and clinical candidates, and also commercially available synthetic MCs, in structure–property space. We find that oral MC drugs occupy defined regions that are distinct from those of the non-oral MC drugs. None of the oral MC regions are effectively sampled by the synthetic MCs. We identify 13 properties that can be used to design synthetic MCs that sample regions overlapping with oral MC drugs. The results advance our understanding of what molecular features are associated with bioactive and orally bioavailable MCs, and illustrate an approach by which synthetic chemists can better evaluate MC designs. We also identify underexplored regions of macrocycle chemical space.


 Please wait while we load your content...
Please wait while we load your content...