An amide-based second coordination sphere promotes the dimer pathway of Mn-catalyzed CO2-to-CO reduction at low overpotential†
Abstract
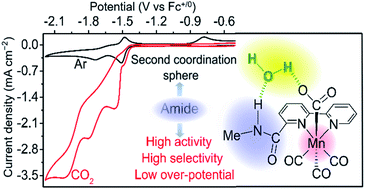
The [fac-Mn(bpy)(CO)3Br] complex is capable of catalyzing the electrochemical reduction of CO2 to CO with high selectivity, moderate activity and large overpotential. Several attempts have been made to lower the overpotential and to enhance the catalytic activity of this complex by manipulating the second-coordination sphere of manganese and using relatively stronger acids to promote the protonation-first pathway. We report herein that the complex [fac-Mn(bpy-CONHMe)(CO)3(MeCN)]+ ([1-MeCN]+; bpy-CONHMe = N-methyl-(2,2′-bipyridine)-6-carboxamide) as a pre-catalyst could catalyze the electrochemical reduction of CO2 to CO with low overpotential and high activity and selectivity. Combined experimental and computational studies reveal that the amide NH group not only decreases the overpotential of the Mn catalyst by promoting the dimer and protonation-first pathways in the presence of H2O but also enhances the CO2 electroreduction activity by facilitating C–OH bond cleavage, making [1-MeCN]+ an efficient CO2 reduction pre-catalyst at low overpotential.


 Please wait while we load your content...
Please wait while we load your content...