Preparation of α-amino acids via Ni-catalyzed reductive vinylation and arylation of α-pivaloyloxy glycine†
Abstract
This work emphasizes easy access to α-vinyl and aryl amino acids via Ni-catalyzed cross-electrophile coupling of bench-stable N-carbonyl-protected α-pivaloyloxy glycine with vinyl/aryl halides and triflates. The protocol permits the synthesis of α-amino acids bearing hindered branched vinyl groups, which remains a challenge using the current methods. On the basis of experimental and DFT studies, simultaneous addition of glycine α-carbon (Gly) radicals to Ni(0) and Ar–Ni(II) may occur, with the former being more favored where oxidative addition of a C(sp2) electrophile to the resultant Gly–Ni(I) intermediate gives a key Gly–Ni(III)–Ar intermediate. The auxiliary chelation of the N-carbonyl oxygen to the Ni center appears to be crucial to stabilize the Gly–Ni(I) intermediate.
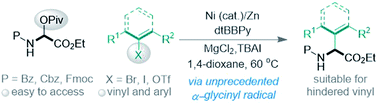


 Please wait while we load your content...
Please wait while we load your content...