Abstract
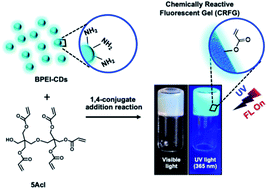
In the past, chemically reactive polymeric interfaces have been considered to be of potential interest for developing functional materials for a wide range of practical applications. Furthermore, the rational incorporation of luminescence properties into such chemically reactive interfaces could provide a basis for extending the horizon of their prospective utility. In this report, a simple catalyst-free chemical approach is introduced to develop a chemically reactive and optically active polymeric gel. Branched-polyethyleneimine (BPEI)-derived, inherently luminescent carbon dots (BPEI-CDs) were covalently crosslinked with pentaacrylate (5Acl) through a 1,4-conjugate addition reaction under ambient conditions. The synthesized polymeric gel was milky white under visible light; however, it displayed fluorescence under UV light. Additionally, the residual acrylate groups in the synthesized fluorescent gel allowed its chemical functionality to be tailored through facile, robust 1,4-conjugate addition reactions with primary-amine-containing small molecules under ambient conditions. The chemical reactivity of the luminescent gel was further employed for a proof-of-concept demonstration of portable and parallel ‘ON’/‘OFF’ toxic chemical sensing (namely, the sensing of nitrite ions as a model analyte). First, the chemically reactive luminescent gel derived from BPEI-CDs was covalently post-modified with aniline for the selective synthesis of a diazo compound in the presence of nitrite ions. During this process, the color of the gel under visible light changed from white to yellow and, thus, the colorimetric mode of the sensor was turned ‘ON’. In parallel, the luminescence of the gel under UV light was quenched, which was denoted as the ‘OFF’ mode of the sensor. This parallel and unambiguous ‘ON’/‘OFF’ sensing of a toxic chemical (nitrite ions, with a detection limit of 3 μM) was also achieved even in presence of other relevant interfering ions and at concentrations well below the permissible limit (65 μM) set by the World Health Organization (WHO). Furthermore, this chemically reactive luminescent gel could be of potential interest in a wide range of basic and applied contexts.


 Please wait while we load your content...
Please wait while we load your content...