A dual-fluorophore sensor approach for ratiometric fluorescence imaging of potassium in living cells†
Abstract
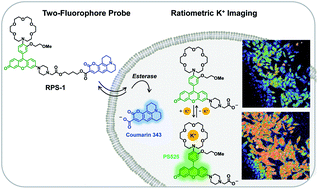
Potassium is the most abundant intracellular metal in the body, playing vital roles in regulating intracellular fluid volume, nutrient transport, and cell-to-cell communication through nerve and muscle contraction. On the other hand, aberrant alterations in K+ homeostasis contribute to a diverse array of diseases spanning cardiovascular and neurological disorders to diabetes to kidney disease to cancer. There is an unmet need for studies of K+ physiology and pathology owing to the large differences in intracellular versus extracellular K+ concentrations ([K+]intra = 150 mM, [K+]extra = 3–5 mM). With a relative dearth of methods to reliably measure dynamic changes in intracellular K+ in biological specimens that meet the dual challenges of low affinity and high selectivity for K+, particularly over Na+, currently available fluorescent K+ sensors are largely optimized with high-affinity receptors that are more amenable for extracellular K+ detection. We report the design, synthesis, and biological evaluation of Ratiometric Potassium Sensor 1 (RPS-1), a dual-fluorophore sensor that enables ratiometric fluorescence imaging of intracellular potassium in living systems. RPS-1 links a potassium-responsive fluorescent sensor fragment (PS525) with a low-affinity, high-selectivity crown ether receptor for K+ to a potassium-insensitive reference fluorophore (Coumarin 343) as an internal calibration standard through ester bonds. Upon intracellular delivery, esterase-directed cleavage splits these two dyes into separate fragments to enable ratiometric detection of K+. RPS-1 responds to K+ in aqueous buffer with high selectivity over competing metal ions and is sensitive to potassium ions at steady-state intracellular levels and can respond to decreases or increases from that basal set point. Moreover, RPS-1 was applied for comparative screening of K+ pools across a panel of different cancer cell lines, revealing elevations in basal intracellular K+ in metastatic breast cancer cell lines vs. normal breast cells. This work provides a unique chemical tool for the study of intracellular potassium dynamics and a starting point for the design of other ratiometric fluorescent sensors based on two-fluorophore approaches that do not rely on FRET or related energy transfer designs.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...