Abstract
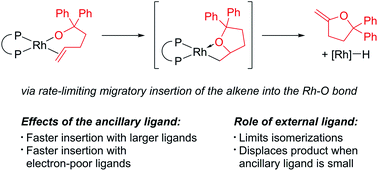
Migratory insertions of olefins into metal–oxygen bonds are elementary steps of important catalytic processes, but well characterised complexes that undergo this reaction are rare, and little information on the effects of ancillary ligands on such reactions has been gained. We report a series of alkoxo alkene complexes of rhodium(I) that contain a range of bidentate ligands and that undergo insertion of the alkene. Our results show that complexes containing less electron-donating ancillary ligands react faster than their counterparts containing more electron-donating ancillary ligands, and that complexes possessing ligands with larger bite angles react faster than those with smaller bite angles. External added ligands had several effects on the reactions, including an inhibition of olefin isomerisation in the product and acceleration of the displacement of the product from complexes of ancillary ligands with small bite angles. Complementary computational studies help elucidate the details of these insertion processes.


 Please wait while we load your content...
Please wait while we load your content...