Cobalt-catalyzed intramolecular decarbonylative coupling of acylindoles and diarylketones through the cleavage of C–C bonds†
Abstract
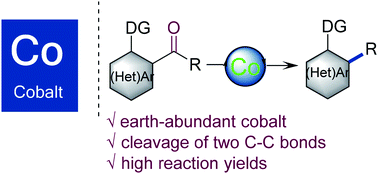
We report here cobalt–N-heterocyclic carbene catalytic systems for the intramolecular decarbonylative coupling through the chelation-assisted C–C bond cleavage of acylindoles and diarylketones. The reaction tolerates a wide range of functional groups such as alkyl, aryl, and heteroaryl groups, giving the decarbonylative products in moderate to excellent yields. This transformation involves the cleavage of two C–C bonds and formation of a new C–C bond without the use of noble metals, thus reinforcing the potential application of decarbonylation as an effective tool for C–C bond formation.


 Please wait while we load your content...
Please wait while we load your content...