Access to P-chiral sec- and tert-phosphine oxides enabled by Le-Phos-catalyzed asymmetric kinetic resolution†
Abstract
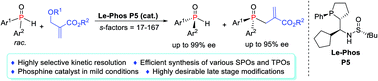
The synthesis of P-stereogenic building blocks is extremely difficult. Herein we report an efficient kinetic resolution of secondary phosphine oxides via a Le-Phos-catalyzed asymmetric allylation reaction with Morita–Baylis–Hillman carbonates. This method provides facile access to enantioenriched secondary and tertiary P-chiral phosphine oxides with broad substrate scope, both of which could serve as P-stereogenic synthons, and can be rapidly incorporated into a given scaffold bearing a P-stereocenter. The highly desirable late stage modifications demonstrate the practicability of our method and can be a critical contribution to obtaining optimal P-chiral catalysts and ligands.


 Please wait while we load your content...
Please wait while we load your content...