Targeted and direct intracellular delivery of native DNAzymes enables highly specific gene silencing
Abstract
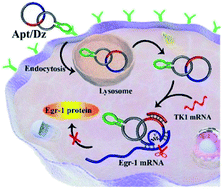
DNAzymes exhibit high potential as gene silencing agents for therapeutic applications. Such purposes, however, are significantly challenged by the targeted and successful delivery of unmodified DNAzymes into cells with minimal side effects. Here, we set out to formulate and demonstrate a new stimuli-responsive and constrained aptamer/DNAzyme (Apt/Dz) catenane nanostructure for highly specific gene silencing. The rational design of the Apt/Dz catenane nanostructure with the respective integration of the aptamer sequence and the completely closed catenane format enables both the targeted capability and significantly improved nuclease resistance, facilitating the stable and targeted delivery of unmodified Dz into cancer cells. Moreover, the Dz enzymatic activity in the constrained structure can only be conditionally regulated by the specific intracellular mRNA sequences to silence the target gene with highly reduced side effects. Results show that the Apt/Dz catenane nanostructure can effectively inhibit the expression of the target gene and the proliferation of cancer cells with high specificity.


 Please wait while we load your content...
Please wait while we load your content...