Reversible cooperative dihydrogen binding and transfer with a bis-phosphenium complex of chromium†
Abstract
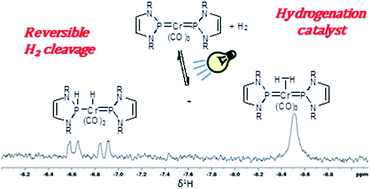
The reversible reaction of H2 with a bis-phosphenium complex of chromium provides a rare example of 3d transition metal/phosphenium cooperativity. Photolysis induces the activation of H2 and yields a spectroscopically detectable phosphenium-stabilized (σ–H2)-complex, readily showing exchange with gaseous H2 and D2. Further reaction of this complex affords a phosphine-functionalized metal hydride, representing a unique example of reversible H2 cleavage across a 3d M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P bond. The same species is also accessible via stepwise H+/H− transfer to the bis-phosphenium complex, and releases H2 upon heating or irradiation. Dihydrogen transfer from the H2-complex to styrene is exploited to demonstrate the first example of promoting hydrogenation with a phosphenium complex.
P bond. The same species is also accessible via stepwise H+/H− transfer to the bis-phosphenium complex, and releases H2 upon heating or irradiation. Dihydrogen transfer from the H2-complex to styrene is exploited to demonstrate the first example of promoting hydrogenation with a phosphenium complex.


 Please wait while we load your content...
Please wait while we load your content...