Photocatalytic Umpolung of N- and O-substituted alkenes for the synthesis of 1,2-amino alcohols and diols†‡
Abstract
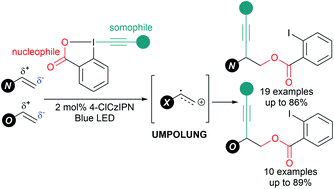
We report an organophotocatalytic 1,2-oxyalkynylation of ene-carbamates and enol ethers using Ethynyl BenziodoXolones (EBXs). 1-Alkynyl-1,2-amino alcohols and diols were obtained in up to 89% yield. Photocatalytic formation of radical cations led to Umpolung of the innate reactivity of the alkenes, enabling addition of a nucleophilic benzoate followed by radical alkynylation.


 Please wait while we load your content...
Please wait while we load your content...