Triazole-based osmium(ii) complexes displaying red/near-IR luminescence: antimicrobial activity and super-resolution imaging†
Abstract
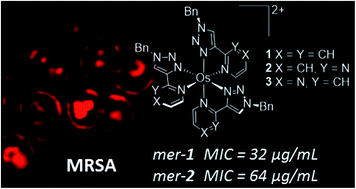
Cellular uptake, luminescence imaging and antimicrobial activity against clinically relevant methicillin-resistant S. aureus (MRSA) bacteria are reported. The osmium(II) complexes [Os(N^N)3]2+ (N^N = 1-benzyl-4-(pyrid-2-yl)-1,2,3-triazole (12+); 1-benzyl-4-(pyrimidin-2-yl)-1,2,3-triazole (22+); 1-benzyl-4-(pyrazin-2-yl)-1,2,3-triazole (32+)) were prepared and isolated as the chloride salts of their meridional and facial isomers. The complexes display prominent spin-forbidden ground state to triplet metal-to-ligand charge transfer (3MLCT) state absorption bands enabling excitation as low as 600 nm for fac/mer-32+ and observation of emission in aqueous solution in the deep-red/near-IR regions of the spectrum. Cellular uptake studies within MRSA cells show antimicrobial activity for 12+ and 22+ with greater toxicity for the meridional isomers in each case and mer-12+ showing the greatest potency (32 μg mL−1 in defined minimal media). Super-resolution imaging experiments demonstrate binding of mer- and fac-12+ to bacterial DNA with high Pearson's colocalisation coefficients (up to 0.95 using DAPI). Phototoxicity studies showed the complexes exhibited a higher antimicrobial activity upon irradiation with light.


 Please wait while we load your content...
Please wait while we load your content...