Nickel-catalyzed migratory alkyl–alkyl cross-coupling reaction†
Abstract
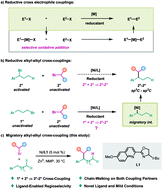
The selective cross-coupling of activated electrophiles with unactivated ones has been regarded as a challenging task in cross-electrophile couplings. Herein we describe a migratory cross-coupling strategy, which can overcome this obstacle to access the desired cross-coupling products. Accordingly, a selective migratory cross-coupling of two alkyl electrophiles has been accomplished by nickel catalysis. Remarkably, this alkyl–alkyl cross-coupling reaction provides a platform to prepare 2°–2° carbon–carbon bonds from 1° and 2° carbon coupling partners. Preliminary mechanistic studies suggest that chain-walking occurs at both alkyl halides in this reaction, thus a catalytic cycle with the key step involving two alkylnickel(II) species is proposed for this transformation.


 Please wait while we load your content...
Please wait while we load your content...