A covalent organic cage compound acting as a supramolecular shadow mask for the regioselective functionalization of C60†‡
Abstract
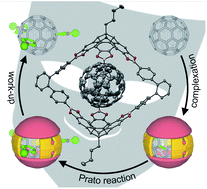
A trigonal-bipyramidal covalent organic cage compound serves as an efficient host to form stable 1 : 1-complexes with C60 and C70. Fullerene encapsulation has been comprehensively studied by NMR and UV/Vis spectroscopy, mass spectrometry as well as single-crystal X-ray diffraction. Exohedral functionalization of encapsulated C60via threefold Prato reaction revealed high selectivity for the symmetry-matched all-trans-3 addition pattern.
- This article is part of the themed collections: Celebrating five years of ChemRxiv and 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...