Palladium catalyzed carbonylative generation of potent, pyridine-based acylating electrophiles for the functionalization of arenes to ketones†
Abstract
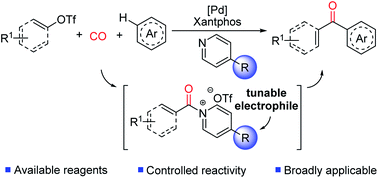
We describe here the design of a palladium catalyzed route to generate aryl ketones via the carbonylative coupling of (hetero)arenes and aryl- or vinyl-triflates. In this, the use of the large bite angle Xantphos ligand on palladium provides a unique avenue to balance the activation of the relatively strong C(sp2)–OTf bond with the ultimate elimination of a new class of potent Friedel–Crafts acylating agent: N-acyl pyridinium salts. The latter can be exploited to modulate reactivity and selectivity in carbonylative arene functionalization chemistry, and allow the efficient synthesis of ketones with a diverse array of (hetero)arenes.


 Please wait while we load your content...
Please wait while we load your content...